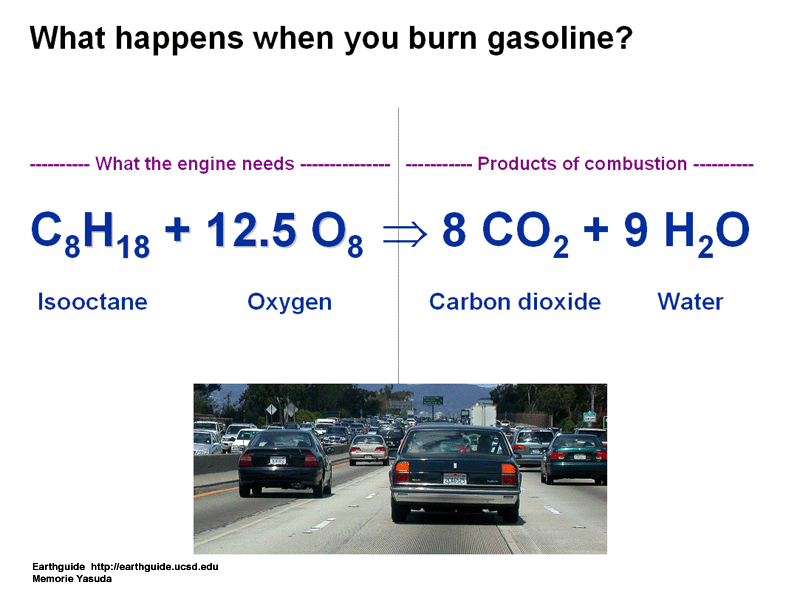
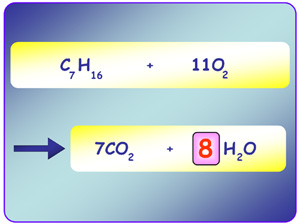
today in class we learned about the ATOMIC THEORY
Early Atomic theory
Greeks history of early atomic theory-In 300 BC, a man named Democritus said atoms were invisible particles, this was the first time the concept of atoms was mentioned
-The problem with this theory was that his theory could not be tested, and it was more of just a model explaining what atoms were thought to be
-Another problem was that there was no mention of the nucleus of any of its constituents
-a third problem was that it couldnt be used to explain chemical reactions, due to the exclusion of valence shell
-somehow, even with all of those problems, his theory was the most veiwed and accepted theory for over 2000 years
<------- basic atomic model of democritus
Lavosier (sometime in the late 1700s)
so later on we have the introduction of a man named Lavosier who introduced a few new things:
--Law of conservation of mass
--Law of definited proportions
- his theory was still not correct because it did not show what atoms were, or how they were arranged
Proust (1799)
now we have the indrocuction of proust some few years later, who more or less backed lavosiers laws and said:
-If a compound is broken down into its constituents, the products exist in the same ratio as in the compound (quote,unquote)
Dalton (early 1800s)
so now a few years later we have the introduction of a man lamed dalton, who introduced quite a few new things:
-he stated that atoms are solid, indestructable spheres, and how they relate the different elements
-a few problems were that he didnt mention isotopes, subatomic particles, or a nucleus

<------ the photo of a genious
J.J Thompson (1850)
so about 40-50 years later we get introduced to yet another scientist, J.J Thompson, who introduced quite a few new things
- He was the first man to introduce ( and is most well known for) his raisin bun model
- this basically stated that the nucleus was a solid positive sphere inside a *membrane* and that the atoms were negative particles embedded in the membrane
- he is the first man to introduce a theory involving positive and negative charges, and is also the first man to mention a nucleus

<-------------------------- raisin bun model
Rutherford (1905)
So something like 55 years later we get introduced to another scientist who worked, reworked and of course, introduced new ideas
- He showed that atoms have a dense center with electrons orbiting it (gold foil experiment)
- these results gave him a more planetary model of the atom, which explains why electrons spin around the nucleus
- He also suggests atoms are mostly empty space
- His problem with that method is that the atoms should attract the electrons, making every element extremely reactive
- He also didnt include valence electrons

<-------- Gold foil experiment
Bohr (1920)
15 years later we get introduced to the man that we refer to the most, and use his theory the most:
- He solved rutherfords problem by stating that electrons must exist in specific orbitals around the nucleus
-he also explained how valence electrons were used in atomic bonding
- he also explained the difference between ionic and covalent bonding
- he also includes the neutron