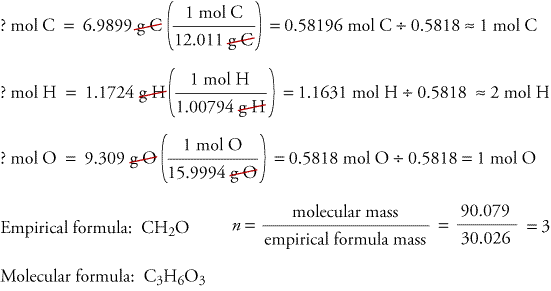
In todays class we learned about Molecular Formula and we did a lab on dilution.
For the lab Mr.Doktor showed us 5 test tubes each that are different colors and we had to compare our test tube to the other 5 and pick one of them.
First we had to measure the amount of water we needed
Then do our calculations to get the amount of Copper (II) Chloride we needed
Then add our solution to the water and stir
Our last step was to pick the test tube that was the most similar to ours.
A Molecular formula is what the equation should actually be.
You will need to know the Empirical Formula before doing the molecular formula lets jut say our empirical formula is CH2O Molecular Formula which is getting the mass in the equation dividing it by the original molar mass and then just multiply the equation and use subscripts.
Example : CH2O has a molecular weight of 180g/mol find the molecular formula.
Original Molar mass = 30g/mol
Mass in equation = 180g/mol
180g/mol / 30g/mol = 6 C = C6 H2 = H12 O = O6
The molecular formula would be C6H12O6
Video showing Molecular formula:
Hi guys. You need to update your blog or give me a new blog address. Remember this is worth 20% of your overall grade.
ReplyDelete