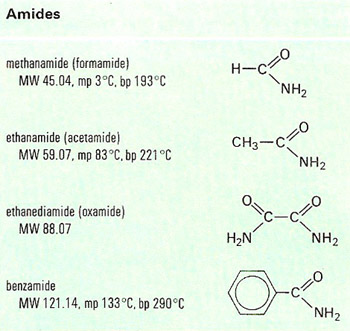
Today we did an esterfication lab, and were tasked to attempt to create a remotely decent smelling ESTER from a alcohol and carboxylic acid.
Here are the list of possible smells we could of created:
isoamyl acetate-----> banana
isobutyl salicylate-----> raspberry
methyl salicylate----->wintergreen <------ the one we wanted
ethyl butyrate-----> pineapple
benzyl butyrate-----> cherry
ethyl propionate-----> rum
isopropyl acetate-----> perfume
benzyl acetate-----> peach
methyl butyrate----->apple
octyl acetate----->orange
propyl acetate-----> pear
ethyl phenylacetate-----> honey
Here are the list of possible smells we could of created:
isoamyl acetate-----> banana
isobutyl salicylate-----> raspberry
methyl salicylate----->wintergreen <------ the one we wanted
ethyl butyrate-----> pineapple
benzyl butyrate-----> cherry
ethyl propionate-----> rum
isopropyl acetate-----> perfume
benzyl acetate-----> peach
methyl butyrate----->apple
octyl acetate----->orange
propyl acetate-----> pear
ethyl phenylacetate-----> honey
So after a lot of effort..err...time, we finally created an Ester, after waiting for it to cool down we wanted to see if it smelled like wintergreen. The smell kind of reminded me of baloons...
After we finished the lab the foods class had to leave to cook for the sports banquet, so the remaining people were given the honor of taking inventory in the shelves.
After we finished the lab the foods class had to leave to cook for the sports banquet, so the remaining people were given the honor of taking inventory in the shelves.