Today in Chemistry class we learned about balancing equations.
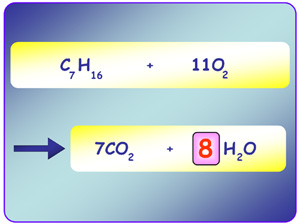
Chemical equations show us the reactants used and the products that are produced during a chemical reaction the numbers in front of the symbols are coefficients which refers to the number of moles.
to balance a chemical equation there must be the same numbers of each atom on both sides of the equation.
Examples :
Al + O2 = Al2O3
C2H6 + O2 = 2CO2 + 3H2O
Fe2O3 + 3H2SO4 = Fe2(SO4)3 + 3H2O
Here is a video showing you how to balance chemical equations:
There are the same number of each type of atom on each side of the equation
No comments:
Post a Comment