Synthesis
Decomposition
Single Replacement
Double Replacement
Neutralization
Combustion
1) Synthesis: two or more substances that are combined
A+b = C
H2 + Cl2 = 2HCL
2)Decomposition: breaking down a compound to simpler elements
AB = A+B
2KClO3 = 2KCL + 3O2
3)Single Replacement: When the compounds have a metal and a non metal and the metals switch places.
A + BC = B + AC
2AGNO3 + CU = CU(NO3)2 + 2AG
4)Double Replacement : exchange of atoms between two different compounds
AB + CD = AD + CB
AgNO3 + KCL = AgCL + KNO3
5)Neutralization: the products will will be water or salt it usually contains an H and OH in the equation balancing out to water
2NaOH + H2CO3 = Na2CO3 + 2H2o
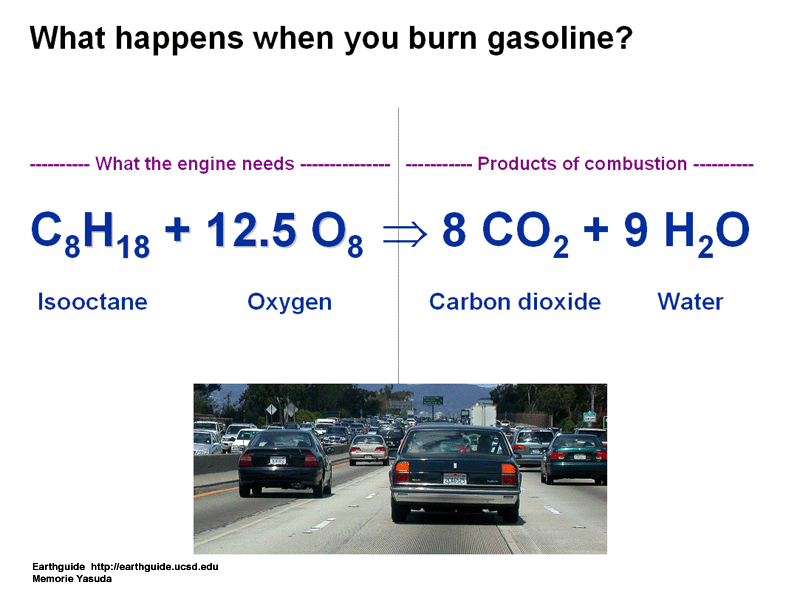
6)Combustion: reacts with oxygen to form an explosion
C3H8 + 5O2 = 3CO2 + 4H2O
Here is a video describing only 5/6 of the chemical reactions. ( it does not describe neutralization)
May you please fix your graphic which shows octane reacting with O8? It should be O2. This graphic is one of the most popular in the world for google searches of "combustion reaction formula" etc and I'm afraid if it isn't taken down or fixed, then it will confuse many students. Thanks! I love your site and am thankful for the work you have put into it! -Lance Culnane- Ph.D. in chemistry and teacher in Colorado. Here is the specific picture I'm talking about:http://earthguide.ucsd.edu/events/TeacherTECH_2005/equation_combustion.gif
ReplyDelete